Marbo-5
Special treatment for respiratory and digestive diseases: Mycoplasma, Pasteurella, infectious atrophic rhinitis, CRD, C.CRD, typhoid, E. coli, head swelling, arthritis




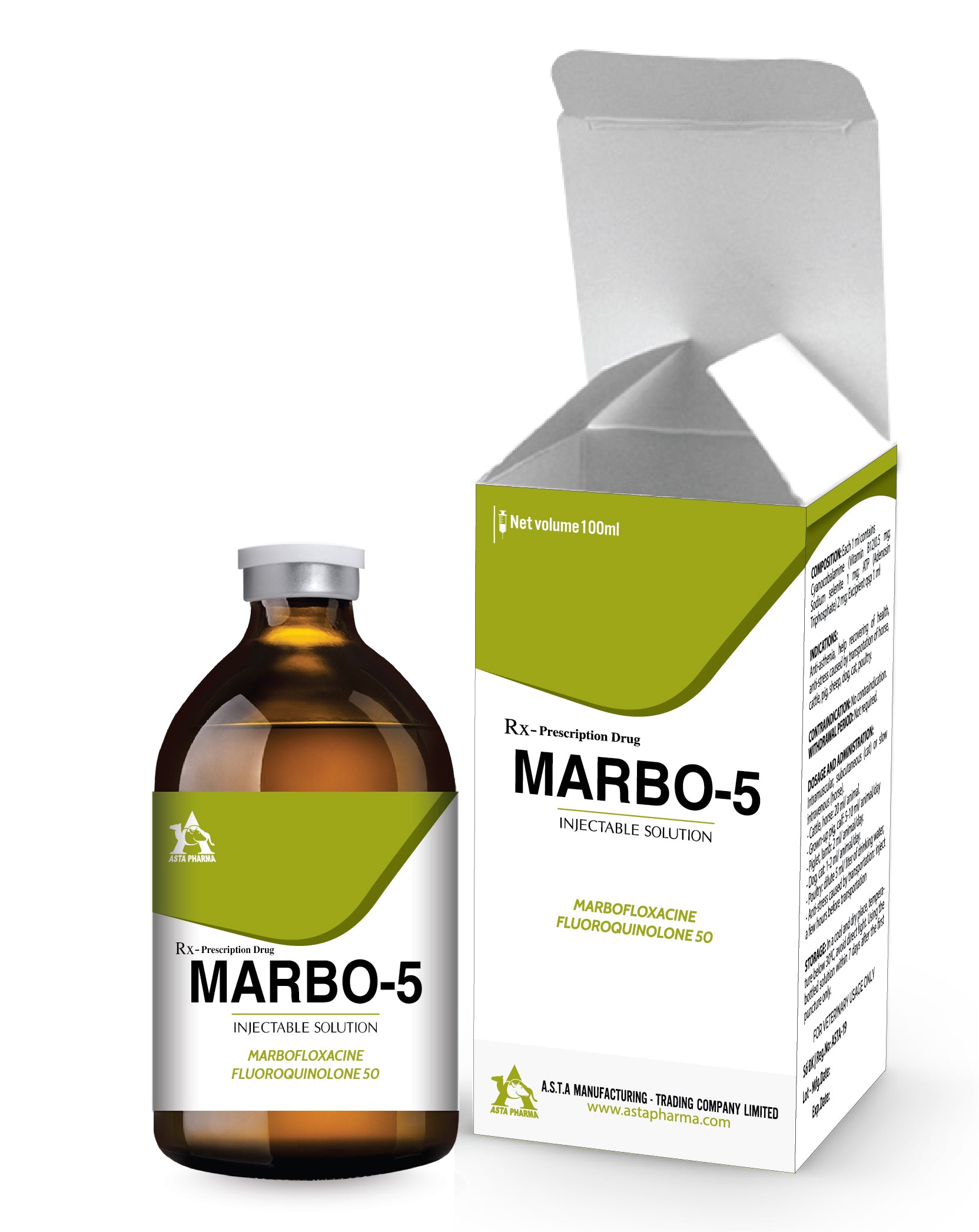

Ingredient
Uses
Pharmacological properties
Usage
Recommendations
Contraindications
Side effects
Expiry date
Storage
Share on
Additional Information
Ingredient
Uses
Pharmacological properties
Usage
Recommendations
Contraindications
Side effects
Expiry date
Storage
Why Choose Us

Trusted Brand
ASTA-Pharma is recognized as a reliable name in the veterinary industry, known for our commitment to animal health.

Diverse Product Range
Our extensive portfolio includes a wide variety of veterinary medicines and solutions to meet diverse needs.
Live Chat
We always have a team of experts to support you 24/7 any weekend and holidays
Our Services
Get in touch
203/2/27, Đ. Đặng Thuỳ Trâm, Phường 13, Bình Thạnh, Hồ Chí Minh
contact@astapharma.net
+84 28 3553 4524
Mon - Fri 8:00 - 17:00 / Sat - Sun off