February 25, 2025
Pharmacological Properties of Tildipirosin in Veterinary Medicine
- huy tran
Overview of Tildipirosin
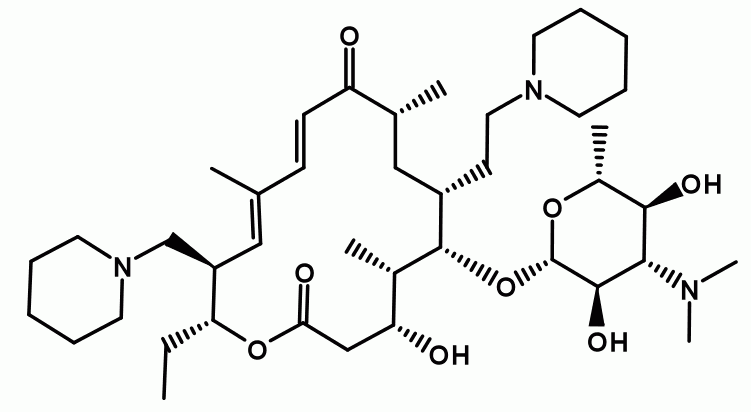
Tildipirosin is a semi-synthetic macrolide derived from tylosin, characterized by a 16-membered lactone ring and three positively charged nitrogen atoms. Specifically engineered for veterinary use, it targets respiratory pathogens in cattle and swine, including bovine respiratory disease (BRD) caused by Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni, and swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae and Haemophilus parasuis. Marketed under the trade name Zuprevo™, Tildipirosin has been rigorously evaluated and approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for its efficacy and safety.
Mechanism of Action
Tildipirosin exerts its antibacterial effect by inhibiting bacterial protein synthesis, a hallmark of macrolides. It binds selectively to the 50S ribosomal subunit at the 23S rRNA region, proximal to the peptidyl-transferase center, thereby blocking polypeptide chain elongation. This action typically confers a bacteriostatic effect, though at higher concentrations particularly against highly susceptible pathogens like M. haemolytica it exhibits bactericidal properties. A seminal study by Andersen et al. (2012) in Antimicrobial Agents and Chemotherapy demonstrated that Tildipirosin’s tri-basic structure enhances ribosomal affinity and cellular penetration compared to congeners like tilmicosin, underpinning its prolonged efficacy.
Pharmacokinetic Profile
Tildipirosin’s pharmacokinetic attributes rapid absorption, extensive tissue distribution, and prolonged half-life are pivotal to its clinical success:
- Absorption: Administered at the recommended dose of 4 mg/kg via subcutaneous (SC) injection in cattle or intramuscular (IM) injection in swine, Tildipirosin achieves peak plasma concentrations (Tmax) within 0.5–1 hour, reflecting swift bioavailability.
- Distribution: With a large volume of distribution (Vz ≈ 5–7 L/kg in cattle), Tildipirosin exhibits exceptional tissue penetration, particularly into the lungs and bronchial secretions the primary sites of respiratory pathogens. Menge et al. (2012), in Journal of Veterinary Pharmacology and Therapeutics, reported pulmonary concentrations 50–100 times higher than plasma levels, exceeding the MIC90 of target pathogens for 16–28 days post-dose.
- Metabolism and Elimination: Minimally metabolized, Tildipirosin is excreted predominantly unchanged via bile and urine. Its elimination half-life (t½) ranges from 17–43 hours, enabling a single-dose regimen with sustained therapeutic activity.
Pharmacodynamic Properties
Tildipirosin’s spectrum of activity is tailored to respiratory pathogens, primarily Gram-positive and select Gram-negative bacteria:
- M. haemolytica (MIC90 ≈ 1 μg/mL)
- P. multocida (MIC90 ≈ 1–2 μg/mL)
- H. somni (MIC90 ≈ 4 μg/mL)
- A. pleuropneumoniae and H. parasuis (MIC90 ≈ 1–4 μg/mL).
Its efficacy correlates with the AUC/MIC ratio (area under the concentration-time curve relative to the minimum inhibitory concentration). Zeng et al. (2018) established in a murine model that an fAUC0-24h/MIC of 19.93 ensures bacteriostasis, 31.89 achieves a 1-log10 CFU reduction, and 53.27 yields a 2-log10 CFU reduction against P. multocida. These PK/PD indices validate the 4 mg/kg dose as optimal for clinical efficacy while minimizing resistance emergence.
Clinical Applications and Real-World Evidence
Tildipirosin is deployed for both treatment and metaphylaxis in high-risk cattle and swine herds. A field trial by Bringhenti et al. (2021) in Journal of Dairy Science demonstrated that prophylactic Tildipirosin in dairy calves significantly reduced BRD and otitis incidence compared to controls, though it did not enhance average daily gain (ADG). In swine, Rose et al. (2016) confirmed sustained antibacterial activity in bronchial secretions against A. pleuropneumoniae for at least 21 days post-administration, underscoring its utility in SRD management.
Advantages and Limitations
Advantages:
- Extended duration of action, reducing administration frequency.
- High pulmonary concentrations, optimizing efficacy at infection sites.
- Reduced cross-resistance with other macrolides due to its unique tri-basic structure.
Limitations:
- Narrow spectrum excludes enteric Gram-negative pathogens (e.g., E. coli).
- Emerging resistance in P. multocida isolates, as noted by Michael et al. (2012) in Europe, necessitates judicious use and MIC monitoring.
Practical Guidance for Veterinary Use
- Dosage: 4 mg/kg, single SC injection in cattle or IM in swine.
- Indications: Treatment and metaphylaxis of BRD and SRD in at-risk populations.
- Withdrawal Periods: In beef cattle, 21–47 days pre-slaughter (varies by jurisdiction); contraindicated in lactating dairy cows over 20 months or veal calves.
- Precautions: Avoid intravenous administration due to cardiovascular toxicity risks; refrain from co-administration with other macrolides to prevent pharmacodynamic interference.
Conclusion
Tildipirosin stands as a cornerstone in veterinary respiratory therapy, blending a potent ribosomal inhibition mechanism with favorable pharmacokinetics and a targeted spectrum. Its single-dose efficacy and tissue tropism make it an invaluable asset, yet its use demands precision to curb resistance a growing concern in modern animal health.