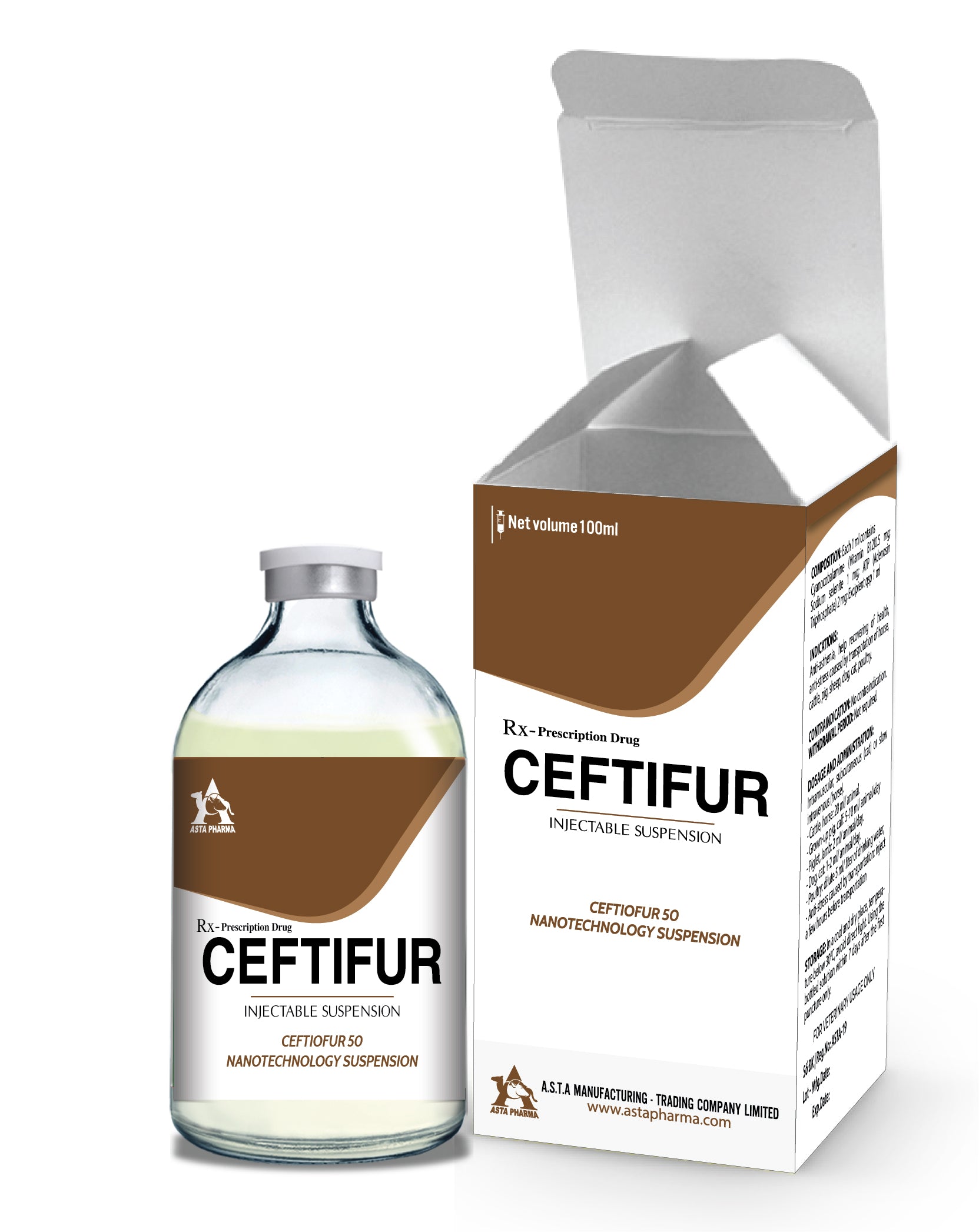
Ceftifur
Ceftiofur 50
NANO-SUSPENSION TECHNOLOGY




RELATED PRODUCTS
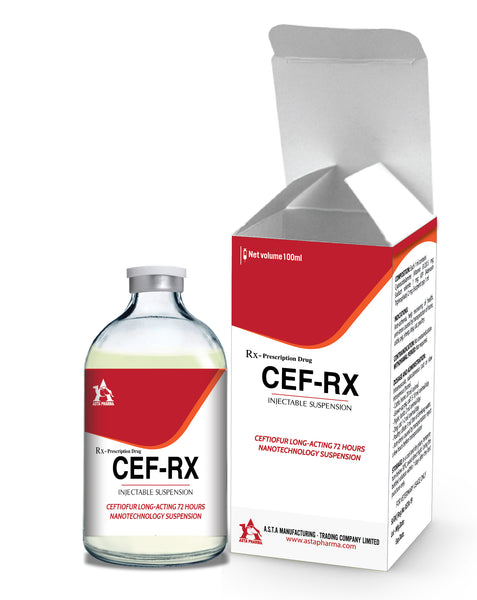
Cef-RX
Treatment of respiratory diseases in pigs

Cefquino
Treatment of respiratory infections, arthritis, and dermatitis in cattle, buffalo, and pigs.

Cefquino-LA
Fourth-generation Cephalosporin With 48-hour Long-acting Effect.Nano-suspension Technology

ClaMOXCIN
Clavulanic acid 1: Amoxicillin 4.NANO TECHNOLOGY SUSPENSION

Co-Amox
Dual Antibiotics.Nano-technology Suspension

Diarstop
A combination of colistin and spectinomycin with synergistic effects.

Galamycin
Treatment of pneumonia, hemolytic E. coli, diarrhea, enteritis, mastitis, metritis, milk reduction

Pig-Cox
Specialized for intracellular developmental stages of Eimeria

Share on
Why Choose Us

ASTA-Pharma is recognized as a reliable name in the veterinary industry, known for our commitment to animal health.

Our extensive portfolio includes a wide variety of veterinary medicines and solutions to meet diverse needs.
We always have a team of experts to support you 24/7 any weekend and holidays
RELATED PRODUCTS

Cef-RX
Treatment of respiratory diseases in pigs

Cefquino
Treatment of respiratory infections, arthritis, and dermatitis in cattle, buffalo, and pigs.

Cefquino-LA
Fourth-generation Cephalosporin With 48-hour Long-acting Effect.Nano-suspension Technology

ClaMOXCIN
Clavulanic acid 1: Amoxicillin 4.NANO TECHNOLOGY SUSPENSION

Co-Amox
Dual Antibiotics.Nano-technology Suspension

Diarstop
A combination of colistin and spectinomycin with synergistic effects.

Galamycin
Treatment of pneumonia, hemolytic E. coli, diarrhea, enteritis, mastitis, metritis, milk reduction

Pig-Cox
Specialized for intracellular developmental stages of Eimeria
